Natural photosynthesis converts CO2 and H2O into hydrocarbons, offering a model for artificial systems aimed at utilizing greenhouse gas CO2 as a resource. Artificial photosynthesis remains challenging, primarily due to the short lifetimes of photogenerated electrons and holes, which hinder synchronized and sustained CO2 reduction and H2O oxidation.
Inspired by natural photosynthesis, the Air Purification New Technology Team (AirPNT) from the Institute of Earth Environment of the Chinese Academy of Sciences, has developed a general approach for converting CO2 and H2O together. Inspired by the role of plastoquinone in temporarily storing electrons during natural photosynthesis, the team design a silver-modified tungsten trioxide (Ag/WO3) that functions as a charge reservoir through reversible W6+/W5+ transitions under irradiation (Fig. 1). This allows the material to store photoexcited electrons and release them on demand, decoupling the two half-reactions in time and enabling better control over the reaction process.
Using this approach, the researchers combined Ag/WO3 with cobalt phthalocyanine (CoPc) to create a composite catalyst. This catalyst achieved a CO production Wrate of approximately 1.5 mmol per gram of CoPc per hour, which is about 100 times higher than that of pure CoPc and matches the performance of systems using organic sacrificial agents.
In addition, the system operates stably under natural sunlight (Fig. 2), offering a practical route to use solar energy for CO2 conversion into clean fuels such as carbon CO and CH4.
This work, published in Nature Communications, was funded by the National Natural Science Foundation of China and the State Key Laboratory of Loess Science.
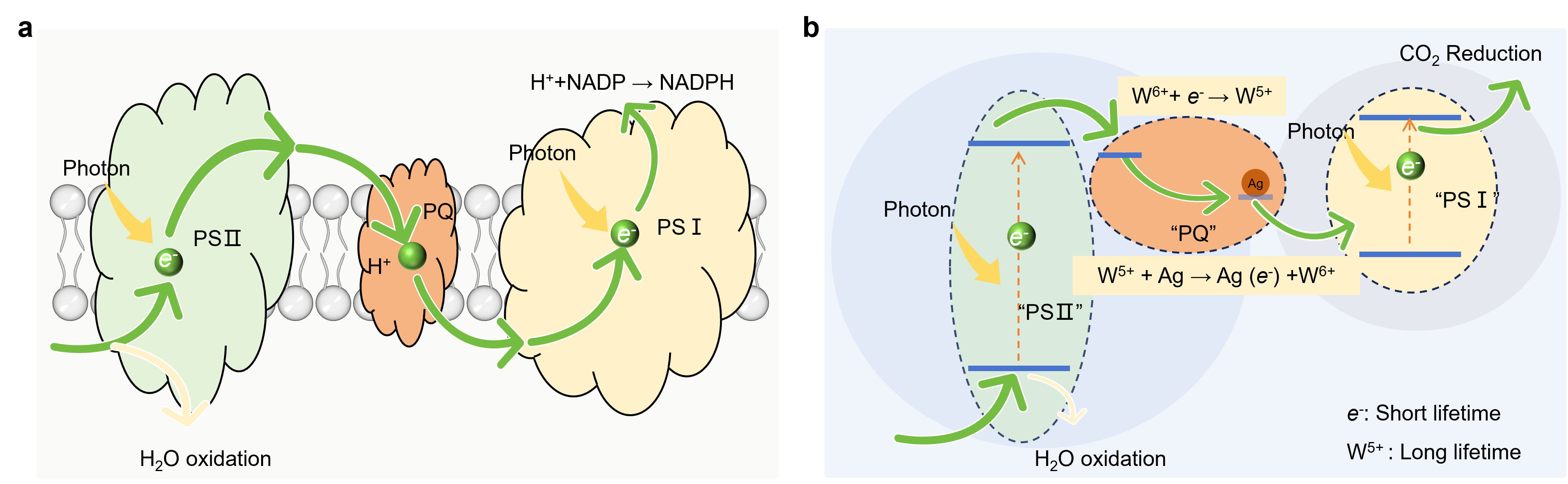
Fig. 1 Electron generation and transfer pathways for sustainable CO2 reduction with H2O. (Image by HUANG, et al)
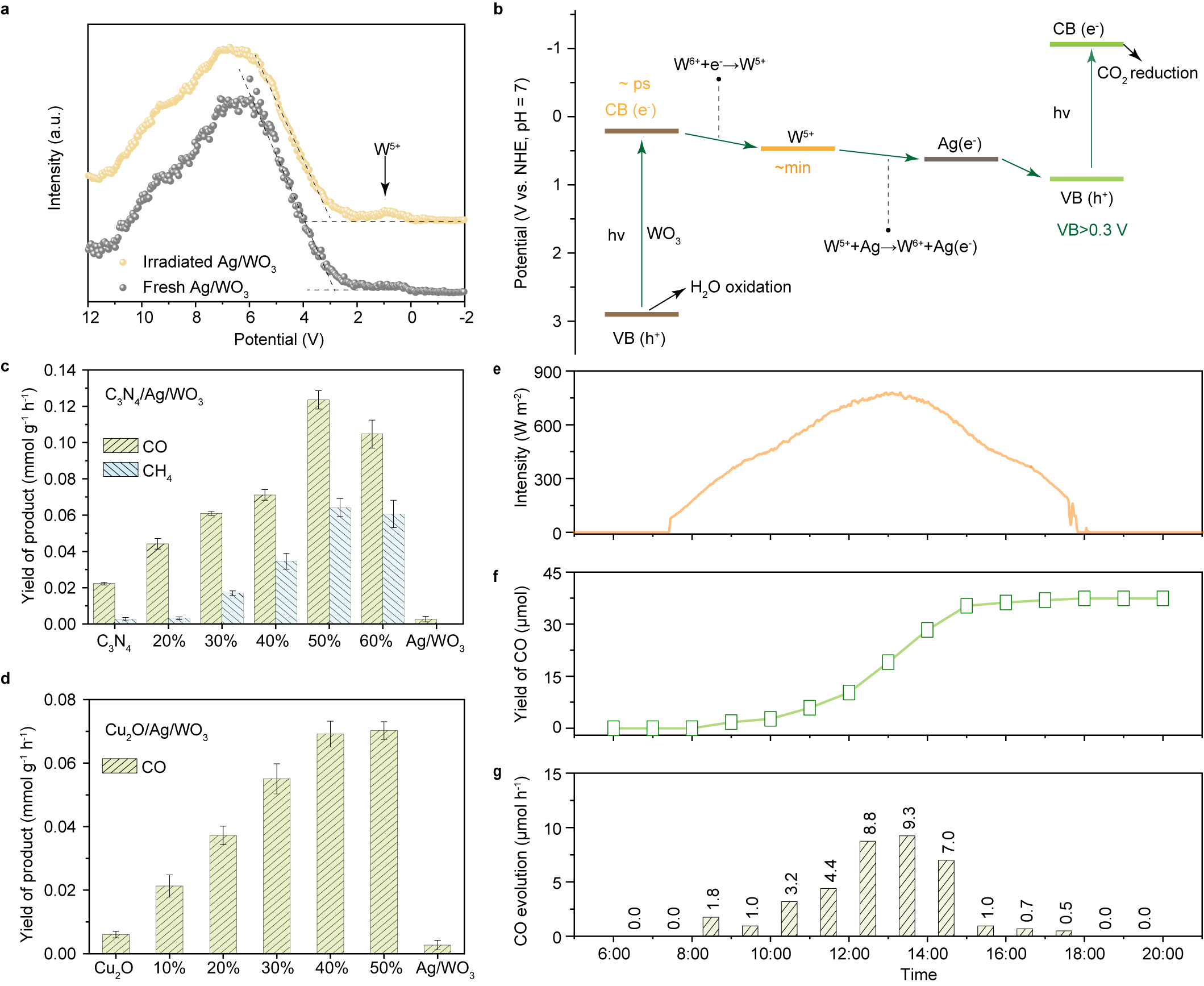
Fig. 2 Demonstration of the universality of the charge reservoir strategy. (Image by HUANG, et al)
 © 2015 Institute of Earth Environment,CAS
© 2015 Institute of Earth Environment,CAS Address:No. 97 Yanxiang Road, Xi'an 710061, Shaanxi, China

 Location :
Location :